Reproduced with permission from The Journal of
Clinical Endocrinology and Metabolism, Vol. 83, No. 1, Copyright 1998 The
Endocrine Society.
The Endocrine Society does not endorse any organization or non-Endocrine Society
products that may appear in this Website. Materials copyrighted by The Endocrine
Society may be reprinted for personal use only. Permission to reprint or
electronically reproduce any document in part or in its entirety for any other
reason is expressly prohibited, unless prior written consent is obtained from
The Endocrine Society.
As published in The Journal of Clinical
Endocrinology and Metabolism Vol. 83, No. 1, pp. 219-223, 1998.
BYUNG-SEOK. LEE, M.D., SOLOMON B. MARGOLIN, PH.D., and ROMANA A. NOWAK. PH.D.
Department of Obstetrics, Gynecology, and Reproductive Biology (B.-S.L., R.A.N.), Harvard Medical School, and Brigham and Women's Hospital, Boston, Massachusetts 02115; and Marnac, Inc. (S.B.M.), Dallas, Texas 75225.
Address all correspondence and requests for reprints to: Dr. Romana A. Nowak, Laboratory of Human Reproduction and Reproductive Biology, Brigham and Women's Hospital, 221 Longwood Avenue, Boston, Massachusetts 02115.
ABSTRACT
There are currently no effective, long term drug therapies for the treatment of
leiomyomas. Pirfenidone (Marnac Inc.) is an antifibrotic agent which is being
tested for use in patients with pulmonary fibrosis. Since leiomyomas are also
characterized by increased cell proliferation and tissue fibrosis, we examined
the effects of pirfenidone on cell proliferation and collagen expression in
cultured myometrial and leiomyoma smooth muscle cells. Effects of pirfenidone on
proliferation of myometrial and leiomyoma cells were measured using tritiated
thymidine incorporation assays and changes in actual cell numbers. Possible
cytotoxic effects were examined using lactate dehydrogenase assays and trypan
blue exclusion. Effects on collagen type I and type III production were assessed
by Northern blotting. Doses of pirfenidone tested were: 0, 0.01, 0.1, 0.3, and
1.0mg/mL. Serum stimulated increases in DNA synthesis and cell proliferation by
myometrial and leiomyoma cells were significantly inhibited in a dose dependent
manner by pirfenidone. Densitometric analysis of Northern blots showed
significantly decreased expression of collagen type I and type III messenger
RNAs in both leiomyoma and myometrial cells. Lactate dehydrogenase (LDH) assays
and trypan blue exclusion measurements showed no cytotoxic effect of pirfenidone
at concentrations that inhibited cell proliferation and collagen production.
Pirfenidone is an effective inhibitor of myometrial and leiomyoma cell
proliferation in vitro and reduces the mRNA levels of collagen types I and III
in a dose-dependent manner. This compound may prove to be an effective
non-steroidal therapy for treatment of uterine leiomyomas.
(J Clin Endocrinol Metab 83: 219-223, 1998.)
_____________________________________________________________________________________
UTERINE leiomyomas or fibroids are the most common pelvic tumors in women with a reported incidence of 20-25% (1). The most common symptoms associated with these benign smooth muscle cell tumors are abnormal uterine bleeding, pelvic pressure or pain, infertility, and increased urinary frequency (2,3). The initiating factor(s) for leiomyomas are not known, however, there is a large body of evidence showing that estrogen and progesterone are important factors for tumor growth (2,4-6). Recent studies have suggested that the effects of these steroid hormones on tumor growth are mediated through the local production of growth factors which exert autocrine or paracrine effects on surrounding cells (7,8).
Gonadotropin-releasing hormone analogs, which reduce serum estradiol and progesterone concentrations to those seen in postmenopausal women, have been used for a number of years as a medical therapy for treatment of leiomyomas (9). However, these compounds are not suitable for long term treatment due to detrimental side effects such as increased loss in bone density (9). Thus there is a need to explore new pharmaceutical agents for the treatment of uterine leiomyomas.
Pirfenidone is an antifibrotic agent which is being investigated for use in patients with pulmonary fibrosis. It is an investigational drug whose structure is 5-methyl-1-phenyl-2-(1H)-pyridone (Fig. 1). Pirfenidone has been shown to produce antifibrotic effects in a variety of animal models (11,12) and to inhibit fibroblast proliferation in vitro in response to a number of growth factors (13).
The goals of the this study were to investigate the effects of pirfenidone on cell proliferation and collagen gene expression in cultured myometrial and leiomyoma cells. We also determined whether this compound had cytotoxic effects on these cells.

FIG. 1. Chemical structure of
pirgenidone (5-methyl-1-phenyl-2(1H)-pyridone)
Subjects and Methods
Patients
Leiomyoma and myometrial tissue were obtained from 12
premenopausal women with symptomatic uterine fibroids at the time of
hysterectomy and who were not receiving any type of hormonal or drug therapy.
Collection of tissues was obtained under a consent for use of discarded human
tissue in accordance with the Brigham and Women's Hospital policy. The stage of
the menstrual cycle for each patient was determined by the pathologist using
endometrial dating. Seven of the patients were in the secretory phase at the
time of surgery, three were in the proliferative phase, and two in the menstrual
phase.
Primary cultures of myometrial and leiomyoma smooth muscle
cells (SMCs) were established as described previously (14). Cultures were
determined to be pure SMC cultures (>98%) by immunostaining for desmin and
smooth muscle alpha-actin which are markers for SMCs (14). Cells were used in
experiments at passages 1 or 2.
Exp 1. For tritiated thymidine incorporation
assays, leiomyoma and myometrial SMCs were cultured in 96-well plates (15,000
cells/well) for 48 h in medium with 10% serum. Cells were then made quiescent by
culturing in medium with 0.5% serum for 48 h. These quiescent cells were washed
and then received medium with 10% serum plus the various doses of pirfenidone
(0, 0.01, 0.1, 0.3, and 1.0 mg/ml). After 18 h the cells received 0.2 uCi/well
of [3H]-thymidine (New England Nuclear, Boston, MA) and the incubation was
continued for a further 6 h. Cells were then harvested and counted to measure
the rate of incorporated [3H]-thymidine. Four experiments using cells from 4
different patients were performed with 6 wells/treatment group/experiment.
Exp 2. In a second set of experiments, myometrial
and leiomyoma SMCs were plated in 100 mm dishes in medium with 10% serum and
cultured until they reached 80-90% confluence. Cells were washed in serum-free
medium, and then placed in serum-free medium containing the various
concentrations of pirfenidone for a period of 3 days. Medium was collected for
assay of lactate dehydrogenase (LDH) levels and cells were harvested and
processed for northern blotting analysis. The LDH assay was used to measure cell
toxicity effects of the various doses of pirfenidone and was performed using a
colorimetric determination kit from Sigma (St. Louis, MO). Four experiments
using cells from 4 different patients were performed with 2 dishes/treatment
group/experiment.
Exp 3. In the third set of experiments, leiomyoma
and myometrial cells were plated in 100 mm dishes (100,000/dish) and allowed to
attach overnight in medium with 10% serum. The following day all cells received
fresh medium with 10% serum containing various concentrations of pirfenidone (0,
0.01, 0.1, 0.3 and 1.0 mg/ml) for a period of 7 days. Medium was changed with
addition of fresh treatments on days 3 and 5. On day 7, cells were harvested ,
counted and viability assessed using the trypan blue exclusion stain. Four
experiments using cells from 4 different patients were performed with 2
dishes/treatment group/experiment.
Total RNA was extracted from culture wells and processed
for northern blot analysis using methods described previously (15). Blots were
probed with a human collagen type I, alpha 1 chain cDNA (ATCC, Rockville, MD), a
human collagen type III, alpha 1 chain cDNA (ATCC) and a human ribosomal
phosphoprotein cDNA (gift of Dr. Dale Goad, Harvard School of Public Health)
using methods established in earlier studies (15,16). [MS1] Filters were
autoradiographed with an intensifying screen for 1-2 days at -70 C. Differences
in sample loading were corrected by normalization to ribosomal phosphoprotein.
Autoradiographs were analyzed on a scanning densitometer (GS-700, Biorad) to
quantitate the levels of transcripts for each sample.
Cell Culture
Experimental Design
Northern Blotting Analysis
Statistics
Statistical analysis was carried out
using two-way analysis of variance (ANOVA). We used contrasts in order to
perform pairwise comparison after the ANOVA procedure. P<0.05 was considered
statistically significant. No significant differences in response to pirfenidone
were noted between cells obtained at different stages of the menstrual cycle.
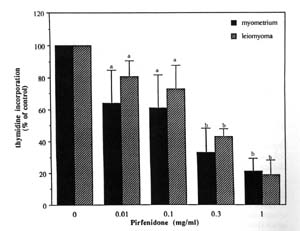
FIG. 2. Effect of pirfenidone on myometrial and
leiomyoma cell proliferation. Concentrations of pirfenidone tested were 0, 0.01,
0.1, 0.3,and 1.0 mg/mL. The effect of the various doses of pirfenidone on
thymidine incorporation is expressed as a percent of control (0 mg/mL
pirfenidone). The serum stimulation of DNA synthesis was significantly inhibited
in a dose dependent manner in both myometrial (P< 0.001) and leiomyoma cells
(P < 0.005). Each bar represents the mean ± SD of 24 wells. Bars bearing
different letters (a vs. b) are significantly different from one another.
Results
The results from the first set of experiments showed that pirfenidone caused a dose dependent inhibition of serum-stimulated DNA synthesis for both normal myometrial and leiomyoma SMCs after 24 h of treatment (Figure 2). Pirfenidone at 0.01mg/ml, 0.1mg/ml , 0.3mg/ml and 1mg/ml inhibited the stimulatory effect of 10% serum to 64+21% (p<0.05), 61+21% (p<0.005), 33%+15% (p<0.001) and 21%+22% (p<0.001) in myometrial cells respectively, and 81+10%(p<0.05), 73%+15% (0.05), 43+4.5% (p<0.001) and 19+10% (p<0.001) in leiomyoma cells respectively. The inhibitory effect of pirfenidone on DNA synthesis does not appear to be due to a toxic effect on the cells since the results of the LDH assay showed no increases in LDH levels in conditioned medium of treated cells at any of the concentrations of pirfenidone (Table 1).
The results of the northen blot analysis for collagen type I mRNA are shown in figure 3. Both 4.8- and 5.8Kb transcripts were detected in every sample. The results showed that mRNA levels of collagen type I were decreased in a dose dependent manner for both myometrial and leiomyoma cells. Pirfenidone at 0.01, 0.1, 0.3 and 1.0 mg/ml reduced collagen type I mRNA levels to 0.82 + 0.11 ADU (p<0.05), 0.66 + 0.16 ADU (p<0.005), 0.56 + 0.21 ADU (p<0.005), and 0.38 + 0.14 ADU (p<0.005) respectively, in myometrial cells, and to 0.92 + 0.1 ADU (p=0.08), 0.67 + 0.16 ADU (p<0.05), 0.6 + 0.15 ADU (p<0.005), and 0.38 + 0.2 ADU (p<0.005) respectively, for leiomyoma cells.
Collagen type III was also down-regulated in a dose dependent manner in myometrial and leiomyoma SMCs (Figure 4) but the effect was more pronounced in myometrial cells. A significant effect of pirfenidone was shown at 0.1mg/ml (0.54+0.22 ADU, p<0.05), 0.3 mg/ml (0.46+0.21ADU, p<0.005) and 1mg/ml (0.41+0.2 ADU, p<0.005) for myometrial cells, whereas in the leiomyoma cells, the collagen type III mRNA levels were significantly inhibited only at the 1 mg/ml concentration (0.54+0.3 ADU, p<0.05).
In the final set of experiments the effects of increasing concentrations of pirfenidone on cell proliferation and cell death after 7 days of treatment were assessed. Results from two representative experiments are shown in Table 2. A significant inhibitory effect on cell proliferation was seen for both leiomyoma and myometrial cells at the 0.1, 0.3 and 1.0 mg/ml concentrations. In contrast, a significant increase in the percentage of dead cells was apparant only at 1.0 mg/ml pirfenidone.
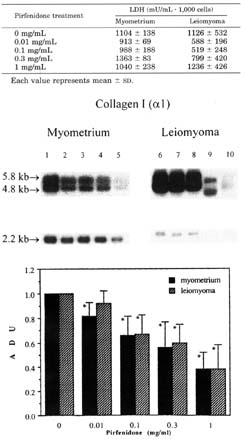
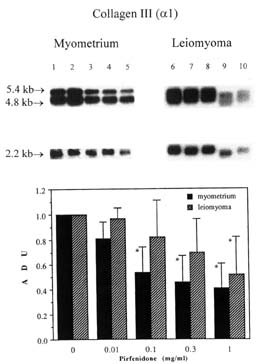
FIG. 4. Effect of pirfenidone on collagen type III (alpha 1) messenger RNA expression in myometrial and leiomyoma cells. Lanes 1,6: 0 mg/mL pirfenidone (P); Lanes, 2,7: 0.01 mg/mL P; Lanes 3,8: 0.1mg/mL P; Lanes 4,9: 0.3 mg/mL P; and lanes 5,10: 1.0 mg/mL P. Two transcripts (4.8 and 5.4 kb) for collagen type III was detected in all lanes. Differences in sample loading were corrected by normalization to ribosomal phosphoprotein (2.2 kb). The graph shows densitometric analysis after normalization. Each bar represents the mean ± SD with * representing P < 0.05.
Discussion
Uterine leiomyomas are a significant health
problem for women but the only effective long term therapy for treatment of
these tumors is hysterectomy or myomectomy. While GnRH agonists have been used
successfully for short term treatment, the detrimental side effects of prolonged
hypoestrogenism have precluded longer term use of these compounds (9). Thus
there is a great need to identify new types of drugs or compounds that may be
effective therapies for leiomyomas but with fewer side effects. In this study we
investigated the effects of the anti-fibrotic compound pirfenidone on uterine
SMC proliferation and matrix protein production. The results show that
pirfenidone is an effective inhibitor of DNA synthesis, cell proliferation and
collagen production for both normal myometrial and leiomyoma SMCs.
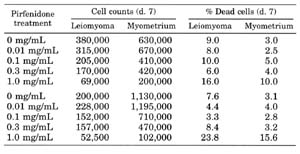 TABLE
2. Effect of pirfenidone on cell proliferation and percentage dead cells.
Results from two representative experiments are shown. Each value represents the
mean of two dishes. Standard errors ranged from 10,000-30,000 cells for cell
counts and from 1-2% for percentage dead cells. Pirfenidone caused a dose
dependent inhibition of cell proliferation for both leiomyoma and myometrial
cells (P < 0.01). A significant increase in percentage dead cells was
apparent only at 1.0 mg/mLpirfenidone (P < 0.05).
TABLE
2. Effect of pirfenidone on cell proliferation and percentage dead cells.
Results from two representative experiments are shown. Each value represents the
mean of two dishes. Standard errors ranged from 10,000-30,000 cells for cell
counts and from 1-2% for percentage dead cells. Pirfenidone caused a dose
dependent inhibition of cell proliferation for both leiomyoma and myometrial
cells (P < 0.01). A significant increase in percentage dead cells was
apparent only at 1.0 mg/mLpirfenidone (P < 0.05).
The mechanism by which pirfenidone acts to inhibit
DNA synthesis and cell proliferation is not clear but it does not appear to
involve toxic effects on the cells. The results of the LDH assay showed no
increase in LDH secretion by cells treated with any of the concentrations of
pirfenidone. In addition, trypan blue exclusion staining performed on cells
treated with the various concentrations of pirfenidone for 7 days showed an
increase in the percentage of dead cells only at the highest concentration of
pirfenidone (1 mg/ml). The inhibitory effect of pirfenidone on cell
proliferation was apparant at one tenth this concentration (0.1 mg/ml). The
relative lack of toxicity of pirfenidone in vitro is supported by findings in
vivo. In clinical studies involving treatment of human subjects with pirfenidone
as a treatment for pulmonary fibrosis, the daily dosage was 2400 mg given orally
(17, Dr. G. Raghu, personal communication). Relatively mild adverse effects
including occasional drowsiness, skin rash or gastric discomfort were noted
(17).
The effects of pirfenidone on cell proliferation are most likely mediated via inhibitory effects on specific growth factors. Studies on human fibroblasts have shown that pirfenidone inhibits basic fibroblast growth factor (bFGF), platelet derived growth factor (PDGF), and transforming growth factor beta (TGFB) stimulated cell proliferation (13). Furthermore, these investigators showed that fibroblasts treated with pirfenidone were unable to exit the G1 phase of the cell cycle. These results suggest a post-receptor site of action for pirfenidone. It is possible that pirfenidone may act as an antiestrogen or an aromatase inhibitor in uterine SMCs due to its chemical structure. However, such a mechanism could only be proven through studies showing that pirfenidone interacts directly with the estrogen receptor.
The mitotic activity of myometrial and leiomyoma SMCs in vivo varies throughout the menstrual cycle suggesting that cell proliferation is regulated by ovarian steroid hormones (18,19). However, studies by a number of investigators have been unable to show consistently a direct stimulatory effect of estradiol or progesterone on proliferation of these cells in vitro (20). This is due in part to the fact that uterine SMCs do not maintain steroid hormone responsiveness for prolonged periods of time when placed in culture. The results of a number of recent studies have led to the hypothesis that the effects of the ovarian steroid hormones on cell proliferation may be mediated indirectly through the activation of autocrine and paracrine peptide growth factors including epidermal growth factor(EGF), insulin-like growth factor I and II (IGF-I and IGF-II) (20-22). Basic FGF, PDGF, and TGFB may also be important regulators of cell proliferation in myometrial and leiomyoma cells (23-25). The anti-proliferative effect of pirfenidone may involve a common, post-receptor site of action as has been suggested from the data on fibroblasts, or may involve inhibition of synthesis of one or more growth factors required for cell proliferation.
Leiomyomas contain large amounts of extracelluar matrix consisting of collagen, proteoglycan, and fibronectin and show increased expression of collagen type I and type III mRNAs (15,26,27). The results of the present study showed that pirfenidone significantly inhibited steady state levels of the mRNAs for both collagen type I and type III in myometrial cells at all concentrations tested. Collagen type I mRNA levels were also significantly inhibited in leiomyoma cells. However, the mRNA level of collagen type III was significantly reduced only at the highest concentration (1mg/ml) of pirfenidone tested. Thus, in leiomyomas, pirfenidone appears to selectively inhibit collagen type I production over that of collagen type III. Fujita et al. (28) reported that the ratio of type III:type I collagen protein was decreased in leiomyomas as compared to the corresponding myometrium due to an increase in collagen type I content and a decrease in collagen type III content in leiomyoma tumors. This suggests an alteration in the normal regulation of collagen production in these tumor cells which may account for the differential effect observed between myometrial and leiomyoma cells. Leiomyoma SMCs may be more resistant to inhibition by specific growth factors. The decrease in steady state levels of mRNAs for the collagens may reflect an inhibitory effect on gene transcription or an increase in mRNA turnover. In vivo studies using the hamster model of artificially induced lung fibrosis have shown that pirfenidone causes a marked inhibition of proline hydroxylase levels (11). This finding suggests that pirfenidone may reduce the availability of the hydroxy proline required for collagen synthesis and may therefore inhibit collagen synthesis at the translational level as well.
In summary, the results of the present studies show that pirfenidone inhibits proliferation of myometrial and leiomyoma SMCs, and significantly suppresses steady state mRNA levels of both collagen type I and collagen type III. Pirfenidone showed little toxic effect on either cell type suggesting that this compound may prove to be an effective therapeutic agent for treatment of leiomyomas with minimal side effects. Studies are underway to investigate more thoroughly the mechanism of action of pirfenidone.
Merrill JA, Creasman WT. 1990 Disorders of the
uterine corpus. In: Scott JR, DiSaia, Hammond CB, Spellacy WN, eds. Danforth's
Obstetrics and Gynecology, 6th ed. Philadelphia; Lippincott; pp.1023-1039.
Buttram VC, Reiter RC. 1981 Uterine leiomyomata:
etiology, symptomatology and management. Fertil Steril. 36:433-445.
Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z,
Peterson HB. 1994 Hysterectomy in the United States, 1988-1990. Obstet
Gynecol. 83:549-555.
Farber M, Conrad S, Heinrichs NL, Herrman WL. 1972
Estradiol binding by fibroid tumors and normal myometrtium. Obstet Gynecol.
40:479-483.
Wilson EA, Yang F, Rees ED. 1980 Estradiol and
progesterone binding in uterine leiomyoma and in normal uterine tissues. Obstet
Gynecol. 55:20-24.
Otubu JA, Buttrum VC, Besch NF, Besch PK. 1982
Unconjugated steroids in leiomyomas and tumor-bearing myometrium. Am J Obstet
Gynecol. 143:130-133.
Lippman ME, Dickson RB, Knabbe C, et al. 1986
Autocrine and paracrine growth regulation of breast cancer. Breast Cancer Res
Treat. 7:59-70.
Fisher DA, Lakshmanan J. 1990 Metabolism and
effects of epidermal growth factor and their related growth factors in mammals.
Endocr Rev. 11:418-442.
Stewart EA, Friedman AJ. 1992 Steroidal treatment
of myomas: preoperative and long term medical therapy. Semin Reprod Endocrinol.
10:344-357.
Iyer SN, Wild JS, Schiedt MJ, Hyde DM, Margolin SB,
Giri SN. 1995 Dietary intake of pirfenidone ameliorates bleomycin-induced
lung fibrosis in hamsters. J Lab Clin Med. 125:779-785.
Shetlar MR, Shetlar CL. 1995 Effect of antifibrosis
drug on the survival of keloid transplants in athymic mice. FASEB. J 9:A967.
Lurton JM, Margolin SB, Raghu G. 1996 Pirfenidone
inhibits the stimulatory effects of pro-fibrotic cytokines on human fibroblasts
in vitro. Am J Respir Crit Care Med. 153:A403.
Nowak RA, Rein MS, Heffner LJ, Friedman AJ, Tashjian Jr
AH. 1993 Production of prolactin by smooth muscle cells cultured from human
uterine fibroid tumors. J Clin Endo Metab. 76:1308-1313.
Stewart EA, Friedman AJ, Peck K, Nowak RA. 1994
Relative overexpression of collagen type I and collagen type III mRNAs by
uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin
Endo Metab. 79:900-906.
Stewart EA, Floor AE, Jain P, Nowak RA. 1995
Increased expression of messenger RNA for collagen type I, collagen type III,
and fibronectin in myometrium of pregnancy. Obstet Gynecol. 86:417-422.
Raghu G, Mageto Y, Lurton J, Limond M. 1997
Treatment of idiopathic pulmonary fibrosis (IPF) with a new antifibrotic drug-
pirfenidone. Resp Crit Care Med. 155:A741.
Tiltman A. 1985 The effect of progestins on the
mitotic activity of uterine fibromyomas. Int J Gynecol Path. 4:89-96.
Kawaguchi K, Fujii S, Konish I, Nanbu Y, Nonogake H,
Mori T. 1989 Mitotic activity in uterine leiomyomas during the menstrual
cycle. Am J Obstet Gynecol. 160:637-641.
Rein MS, Nowak RA. 1992 Biology of uterine myomas
and myometrium in vitro. Semin Reprod Endocrinol. 10:310-319.
Harrison-Woolrych ML, Charnock-Jones DS, Smith SK. 1994
Quantification of messenger ribonucleic acid for epidermal growth factor in
human myometrium and leiomyoma using reverse transcriptase polymerase chain
reaction. J Clin Endocrinol Metab. 78:1179-84.
Vollenhoven BJ, Herington AC, Hearly DL. 1993
Messenger ribonucleic acid expression of the insulin-like growth factors and
their binding proteins in uterine fibroids and myometrium. J Clin Endocrinol
Metab. 76:1106-1110.
Rauk, PN, Surti U, Roberts JM, Michalopoulos. 1995
Mitogenic effect of basic fibroblast growth factor and estradiol on cultured
human myometrial and leiomyoma cells. Am J Obstet Gynecol. 173:571-577.
Stewart EA, Nowak RA. 1996 Leiomyoma-related
bleeding: a classic hypothesis updated for the molecular era. Hum Reprod Update
2:295-306.
Arici A, Sozen I, Olive D. 1995 Transforming growth
factor beta 3 promotes proliferation of myometrial and leiomyoma cells. J Soc
Gynecol Invest. 2:411.
Ferenczy A, Richart RM, Okagaki T. 1971 A
comparative ultrastructural study of leiomyosarcoma, cellular leiomyoma, and
leiomyoma of uterus. Cancer 28:1004-1018.
Kawaguchi K, Fujii S, Konishi I, Okamura H, Mori T. 1985
Ultrastructual study of cultured smooth muscle cells from uterine leiomyoma and
myometrium under the influence of sex steroids. Gynecol Oncol. 21:32-41.
Fujita M. 1985 Histological and biochemical studies
of collagen in human uterine leiomyomas [English abstract]. Hokkaido Igaku
Zasshi. 60:602-615.
References