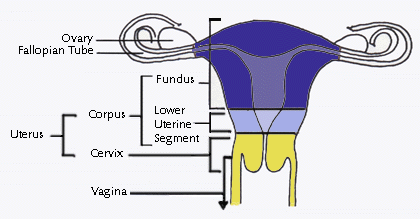
 The
human uterus is a pear-shaped organ composed of two distinct anatomic
regions: the cervix and the corpus. The corpus is further divided into
the lower uterine segment and the fundus. The cervix is a narrow
cylindrical passage which connects at its lower end with the vagina. At
its upper end, the cervix widens to form the lower uterine segment
(isthmus); the lower uterine segment in turn widens into the uterine
fundus. The corpus is the body of the uterus which grows during
pregnancy to carry a fetus. Extending from the top of the uterus on
either side are the fallopian tubes (oviducts); these tubes are
continuous with the uterine cavity and allow the passage of an ova (egg)
from the ovaries to the uterus where the egg may implant if fertilized The
human uterus is a pear-shaped organ composed of two distinct anatomic
regions: the cervix and the corpus. The corpus is further divided into
the lower uterine segment and the fundus. The cervix is a narrow
cylindrical passage which connects at its lower end with the vagina. At
its upper end, the cervix widens to form the lower uterine segment
(isthmus); the lower uterine segment in turn widens into the uterine
fundus. The corpus is the body of the uterus which grows during
pregnancy to carry a fetus. Extending from the top of the uterus on
either side are the fallopian tubes (oviducts); these tubes are
continuous with the uterine cavity and allow the passage of an ova (egg)
from the ovaries to the uterus where the egg may implant if fertilized
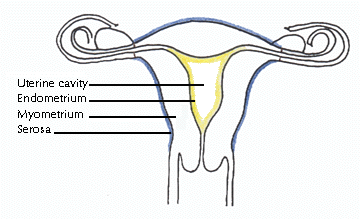 The thick
wall of the uterus is formed of three layers: endometrium, myometrium,
and serosa. The endometrium (uterine mucosa) is the innermost layer that
lines the cavity of the uterus. Throughout the menstrual cycle, the
endometrium grows progressively thicker with a rich blood supply to
prepare the uterus for potential implantation of an embryo. In the
absence of implantation, a portion of this layer is shed during
menstruation. The myometrium is the middle and thickest layer of the
uterus and is composed of smooth (involuntary) muscle. The myometrium
contracts during menstruation to help expel the sloughed endometrial
lining and during childbirth to propel the fetus out of the uterus. The
outermost layer, or serosa, is a thin fibrous layer contiguous with
extrauterine connective tissue structures such as ligaments that give
mechanical support to the uterus within the pelvic cavity. The thick
wall of the uterus is formed of three layers: endometrium, myometrium,
and serosa. The endometrium (uterine mucosa) is the innermost layer that
lines the cavity of the uterus. Throughout the menstrual cycle, the
endometrium grows progressively thicker with a rich blood supply to
prepare the uterus for potential implantation of an embryo. In the
absence of implantation, a portion of this layer is shed during
menstruation. The myometrium is the middle and thickest layer of the
uterus and is composed of smooth (involuntary) muscle. The myometrium
contracts during menstruation to help expel the sloughed endometrial
lining and during childbirth to propel the fetus out of the uterus. The
outermost layer, or serosa, is a thin fibrous layer contiguous with
extrauterine connective tissue structures such as ligaments that give
mechanical support to the uterus within the pelvic cavity.
Non-pregnant uterine size
varies with age and number of pregnancies, but is approximately three
and a half inches long and weighs about one sixth of a pound.
References
-
Cotran, RS, Kumar, V,
and Robbins, SL. Robbins Pathologic Basis of Disease, Fifth Edition.
W.B. Saunders Company, 1994.
-
Netter, FH. Atlas of
Human Anatomy, Sixth Edition. CIBA-GEIGY Corporation, 1993.
Back to top
|
Uterine leiomyomas,
commonly known as fibroids, are well-circumscribed, non-cancerous tumors
arising from the myometrium (smooth muscle layer) of the uterus. In
addition to smooth muscle, leiomyomas are also composed of extracellular
matrix (i.e., collagen, proteoglycan, fibronectin). Other names for
these tumors include fibromyomas, fibromas, myofibromas, and myomas.
Leiomyomas are the most
common solid pelvic tumor in women, causing symptoms in approximately
25% of reproductive age women. However, with careful pathologic
inspection of the uterus, the overall prevalence of leiomyomas increases
to over 70%, because leiomyomas can be present but not symptomatic in
many women. The average affected uterus has six to seven fibroids.
Leiomyomas are usually
detected in women in their 30's and 40's and will shrink after menopause
in the absence of post-menopausal estrogen replacement therapy. They are
two to five times more prevalent in black women than white women. Risk
for developing leiomyomas is also higher in women who are heavy for
their height and is lower in women who are smokers and in women who have
given birth. Although the high estrogen levels in oral contraceptive
pills has led some clinicians to advise women with leiomyomas to avoid
using them, there is good epidemiologic evidence to suggest that oral
contraceptive use decreases the risk of leiomyomas.
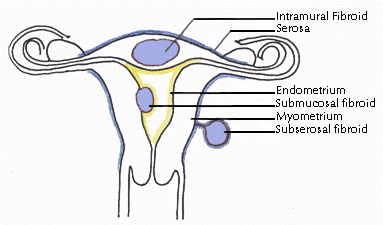
 Leiomyomas
are classified by their location in the uterus. Subserosal
leiomyomas are located just under the uterine serosa and may be pedunculated
(attached to the corpus by a narrow stalk) or sessile
(broad-based). Intramural leiomyomas are found predominantly
within the thick myometrium but may distort the uterine cavity or cause
an irregular external uterine contour. Submucous leiomyomas are
located just under the uterine mucosa (endometrium) and, like subserosal
leiomyomas, may be either pedunculated or sessile. Tumors in subserosal
and intramural locations comprise the majority (95%) of all leiomyomas;
submucous leiomyomas make up the remaining 5%. Leiomyomas
are classified by their location in the uterus. Subserosal
leiomyomas are located just under the uterine serosa and may be pedunculated
(attached to the corpus by a narrow stalk) or sessile
(broad-based). Intramural leiomyomas are found predominantly
within the thick myometrium but may distort the uterine cavity or cause
an irregular external uterine contour. Submucous leiomyomas are
located just under the uterine mucosa (endometrium) and, like subserosal
leiomyomas, may be either pedunculated or sessile. Tumors in subserosal
and intramural locations comprise the majority (95%) of all leiomyomas;
submucous leiomyomas make up the remaining 5%.
Although this classification
scheme is widely used by clinicians, it suffers from the limitation that
few leiomyomas are actually a single "pure" type. Most
leiomyomas span more than one anatomic location and, therefore, are hybrids
(e.g., a predominantly intramural leiomyoma with a submucous component).
Other types of leiomyomas include "parasitic" myomas, which
receive their blood supply from structures other than the uterus (e.g.,
the omentum), and seedling myomas, which have a diameter of less than or
equal to four millimeters.
Transformation of uterine
leiomyomas (benign) to uterine leiomyosarcomas (malignant smooth
muscle tumors of the uterus) is extremely rare, and, in fact, many
researchers and clinicians believe this type of transformation never
occurs. However, without pathologic examination of the uterus, this
determination is not possible. Uterine leiomyosarcomas are found in
approximately 0.1% of women with leiomyomas and are reported to be more
frequently associated with large or rapidly growing fibroids. Therefore,
surgical intervention may be undertaken in women with these types of
tumors to rule out leiomyosarcoma, a rare but medically important
lesion.
Back to top
|
Research indicates that
between 20% and 50% of women have fibroid-related symptoms. The two most
common symptoms of fibroids (also called leiomyomas) are abnormal
uterine bleeding and pelvic pressure.
The most common bleeding
abnormality is menorrhagia (prolonged and/or profuse uterine
bleeding, also called hypermenorrhea). Normal menstrual periods
typically last four to five days, whereas women with fibroids often have
periods lasting longer than seven days. Women with fibroids also can
have such heavy bleeding that they need to change sanitary protection
frequently (perhaps every hour) or hesitate to participate in their
normal activities for fear of socially embarrassing bleeding. Bleeding
between periods is not usually associated with fibroids and should
always be investigated by a physician. Although abnormal bleeding can
occur with any of the three classes of fibroids,
women with submucous fibroids seem particularly prone to this
complication.
Pelvic pressure results from
an increase in size of the uterus or from a particular fibroid. Most
women with leiomyomas have an enlarged uterus; in fact, doctors describe
the size of a uterus with fibroids as they would a pregnant uterus, for
example, as a 12 week-size fibroid uterus. It is not unusual for a
uterus with leiomyomas to reach the size of a four to five month
pregnancy. In addition to vague feelings of pressure because a fibroid
uterus is usually irregularly shaped (having many lumps and bumps),
women can experience pressure on specific adjacent pelvic structures
including the bowel and/or bladder. Pressure on these structures can
result in difficulty with bowel movements and constipation or urinary
frequency and incontinence. Rarely, fibroids can press on the ureters
(which carry urine from the kidneys to the bladder) which can lead to
kidney dysfunction.
Leiomyomas are also
associated with a range of reproductive dysfunction including
recurrent miscarriage, infertility, premature labor, fetal
malpresentations, and complications of labor. Although few studies exist
regarding fibroid-related reproductive dysfunction, the prevailing
clinical perspective is that these complications most often occur when
fibroids physically distort the uterine cavity. Therefore, women with
large or symptomatic fibroids may choose to undergo assessment of the
uterine cavity (such as by hysterosalpingograpy or by hysteroscopy, see
below) before attempting pregnancy. If fibroids are detected on the
inside of the uterus (termed submucous fibroids) and
distort the uterine lining, they are a significant cause of reproductive
problems and should be removed. It is less clear whether fibroids in the
wall of the uterus cause reproductive problems. Generally, if the uterus
is small, fibroids do not need to be removed in women contemplating or
attempting pregnancy.
The diagnosis of leiomyomas
is usually easily determined by bimanual pelvic examination.
During this routine office exam, the clinician evaluates the size and
shape of the uterus and surrounding pelvic structures by inserting two
fingers of one hand into the vagina while palpating the patient's
abdomen above the pubic bone with the other hand. During this exam, a
uterus with fibroids often feels enlarged and/or irregular and may be
felt abdominally above the pubic bone. In contrast, a non-pregnant
uterus without fibroids is not palpable above the pubic bone.
In addition, imaging studies
such as ultrasonography, MRI (magnetic resonance imagery),
and CT (computed tomography) may be useful in confirming the
diagnosis. Currently, ultrasonography is the most common method of
confirming the diagnosis of leiomyomas, but MRI may prove to be the most
useful method because it can often distinguish leiomyomas from other
intramural lesions.
In patients experiencing
menorrhagia (profuse and/or prolonged menstrual flow) or recurrent
pregnancy losses, assessment of the uterine cavity is important
because the presence of a submucous fibroid can be missed on traditional
ultrasound.
Hysterosalpingography, sonohysterography, and hysteroscopy
can all supply this information and aid in a more definitive diagnosis of
fibroids. More invasive procedures such as laparoscopy can also aid in
definitive diagnosis. Hysterosalpingography and
sonohysterosgraphy use X-ray pictures and ultrasound pictures,
respectively, to visualize the uterine cavity after a specific dye is
injected into the uterus. Hysteroscopy allows direct visualization
of the uterine cavity by inserting a small camera on the end of a long tube
(hysteroscope) directly into the uterus through the vagina and cervix.
Laparoscopy, on the other hand, allows direct visualization of the
outside of the uterus and the surrounding pelvic structures by introducing
a small camera on the end of a tube (laparoscope) directly into the
abdominal cavity.
Back to top
|
In general, fibroids only
need to be treated if they are causing symptoms. The primary treatment
for patients with large or symptomatic fibroids is surgery. Hysterectomy
(surgical removal of the entire uterus) is the most frequent operative
technique used to treat this disorder. In fact, fibroids are the most
common indication for hysterectomy, accounting for approximately one
third of hysterectomies, or about 200,000 procedures annually, in the
United States.
There are a variety of types
of hysterectomy including abdominal hysterectomy , vaginal
hysterectomy, supracervical hysterectomy, and laparoscopically-assisted
vaginal hysterectomy. The type of hysterectomy chosen depends on the
size of the uterus, the woman's medical history, and the skills of her
surgeon. The advantage of hysterectomy in the treatment of leiomyomas is
that it provides a true "cure" for fibroids, but is only an
option for women who are not planning future pregnancies.
When women wish to preserve
childbearing potential, a myomectomy may be performed. Unlike
hysterectomy in which the entire uterus is removed, myomectomy is a
surgical procedure in which individual fibroid(s) are removed.
Approximately 18,000 myomectomies are performed yearly in the United
States. Most myomectomies are performed through an abdominal incision,
although certain submucous fibroids can be removed
through the vagina without an abdominal incision during a procedure
called hysterosopic myomectomy which involves a special
instrument called a hysteroscope. This technique is
primarily useful for women with bleeding or pregnancy-related problems
as there is usually little change in the size of the uterus with this
approach. Certain subserosal fibroids may be removed
abdominally during a procedure called laparoscopic myomectomy
which involves a different instrument called a laparoscope.
In general, myomectomy diminishes menorrhagia (prolonged and/or profuse
menstrual flow) in roughly 80% of patients presenting with this symptom.
Unfortunately, there is a significant risk of recurrence of
fibroids after myomectomy; in some studies up to 10% of women who
underwent an initial myomectomy required a second major operative
procedure. In addition, a quarter to a half of women who underwent
myomectomies had evidence by ultrasound of recurrence of their fibroids
within one to ten years.
There are also several
innovative techniques being studied as possible surgical treatment for
fibroid-related bleeding. Myolysis involves delivering electric
current via needles to a fibroid at the time of laparoscopy.
Cryomyolysis involves using a freezing probe in a similar manner.
Uterine artery embolization is a radiological alternative to
surgery that involves placing a catheter into an artery in the leg and
guiding the catheter via x-ray pictures to the arteries of the uterus.
Once there, the catheter is used to deliver agents that block off these
major blood vessels. While all of these treatments may prove to be
effective treatments for fibroids, compared to more traditional options,
the number of patients treated by these methods have been small, the
follow-up relatively short term, and the safety of these procedures in
women desiring pregnancy has not been demonstrated.
Back to top
|
Medicines can help control
fibroid-related symptoms. The most effective medications for the
treatment of fibroids are gonadotropin releasing
hormone agonists (GnRHa), (including Lupron, Synarel, Zoladex).
GnRH agonists induce a low-estrogen (menopause-like) state. Because
fibroids are dependent on estrogen for their development and growth,
induction of a low estrogen state causes reduction of tumor and
uterus mass, resolving pressure symptoms. (Specifically, uterine
volume has been shown to decrease approximately 50% after three months
of GnRH agonist therapy.) In addition to decreasing the size of the
uterus, treatment with GnRH agonists also stops menstrual flow (amenorrhea),
allowing women with bleeding-induced anemia to significantly increase
their iron stores. Unfortunately, cessation of GnRH agonist treatment is
followed by a rapid regrowth of the fibroids and of the uterus to
pre-treatment volume. Additionally, because bone also requires estrogen,
long term use of GnRH agonists can significantly decrease bone density
and can lead to bone loss or osteoporosis. Currently, therefore,
use of GnRH agonists alone for treatment of fibroids is usually limited
to a short one to three month preoperative course to shrink the uterus
to facilitate a surgical procedure or to induce amenorrhea to improve
hematologic condition before surgery.
The combination of
GnRH agonists and low doses of the steroid hormones estrogen and
progesterone (i.e., "add-back" regimens) has been employed in
some clinical trials in an attempt to safely extend the maximum duration
of GnRH agonist therapy without sacrificing efficacy. These regimens
have been studied for use of up to two years. Preliminary data suggest
they may be safe and effective if the hormone dose is low (equivalent to
menopausal replacement doses versus high dose birth control pills) and
if the GnRH-agonist is given alone first, allowing the uterus to shrink
before the hormones are added. This approach appears to maintain uterine
shrinkage and/or control of bleeding while supporting other tissues such
as bone and minimizing side effects such as hot flashes that accompany
the low estrogen levels induced by GnRH-agonist therapy alone.
Several innovative options
are under investigation as possible future medical treatments for
uterine leiomyomas. There have been several small studies examining the
use of GnRH antagonists in leiomyomas. The primary advantage of
antagonists over more widely used GnRH agonists is that antagonists have
a faster onset of action. However for long-term therapy, antagonists
appear to have no advantage. The progesterone antagonist,
mifepristone (RU 486), has also been shown in small studies to induce
uterine shrinkage and stop menstrual periods in women with fibroids.
However, this agent is not currently available in the United States.
Studies of the antifibrotic drug, pirfenidone, are also underway
to determine if this agent is useful in the treatment of fibroids.
Other medical therapies
including androgenic agents (e.g., danazol, gestrinone), progestins
(e.g., medroxyprogesterone acetate, depomedroxyprogesterone acetate,
norethindrone), and oral contraceptive pills have also been used
to control menorrhagia (prolonged and/or profuse blood flow) in women
with leiomyomas, presumably by diminishing the endometrium (endometrial
atrophy). However, these medications do not consistently decrease uterus
or fibroid volume and are often ineffective in controlling menorrhagia.
Back to top
|
The Food and Drug Administration (FDA) approved the ExAblate 2000 technology for focused ultrasound treatment of uterine fibroids on Oct 22, 2004. The Center for Uterine Fibroids at Brigham and Women's Hospital has a commercial treatment program using MR guided Focused Ultrasound. This treatment is an outpatient procedure designed to reduce fibroid related symptoms. The Center is actively working with insurers to obtain insurance coverage for this treatment. Women who are interested in commercial treatment can contact us at (800) BWH-9999.
The following information may be useful to you:
Back to top
|
Despite the major public
health impact of leiomyomas, little is known about their cause. Until
recently, the steroid hormones estrogen and progesterone were
considered the most important regulators of leiomyoma growth. There is
abundant evidence that estrogen promotes fibroid growth including
the clinical observations that fibroids grow in the presence of high
levels of estrogen, such as during the reproductive years, and that they
regress in the presence of low levels of estrogen, such as following
menopause or during gonadotropin releasing hormone (GnRH) agonist
therapy. Furthermore, fibroids have higher estrogen concentrations, bind
more estrogen, have more estrogen receptors, and convert estradiol (a
more active form of estrogen) to estrone (a less active form of
estrogen) more slowly than normal myometrium.
Progesterone
is also thought to play a role in fibroid growth. More specifically,
clinical studies suggest progesterone facilitates the growth of
fibroids. For example, fibroid size increases during treatment with
synthetic progesterones. Combination GnRH agonist and progesterone
therapy has been shown to have no effect on uterine volume, in contrast
to GnRH agonist therapy alone which has been shown to reduce uterine
volume. The observation that fibroids regress with the antiprogesterone
agent, RU-486, further supports the role of progesterone as a promoter
of fibroid growth. Histologically, fibroids from patients treated with
progesterone show more cellular growth than those from patients without
progesterone therapy. Biochemically, fibroids have higher progesterone
receptor concentrations than normal myometrium. Together, these data
suggest that progesterone also enhances fibroid growth.
Other hormones such as growth
hormone (GH) and prolactin (PRL) are also thought to promote
fibroid growth, but their role is even less well defined.
More recently, growth
factors, which are small proteins that affect cell growth, have been
shown to mediate the growth-promoting effects of estrogen and to play an
important role in the development of fibroid tumors. Potentially
important factors in fibroid growth include transforming growth
factor-beta, basic fibroblast growth factor, epidermal growth factor,
insulin-like growth factor, and platelet-derived growth factor. (For
more information about this, please see the article, Leiomyoma-related
bleeding: A classic hypothesis updated for the molecular era, on the
"Publications" page.)
Overall, estrogen,
progesterone, and growth factors likely promote tumor growth, but only
after the initiation of tumor formation. This initiating event remains
unknown, although recent evidence suggests there is a strong inherited
component to fibroid development. Indirect evidence for this
hypothesis is as follows. First, fibroids are at least twice as common
in black women than in white women. Although racial differences in
socioeconomic status and access to health care, as well as racial
differences in known risk factors for fibroids, may contribute to this
finding, two recent studies suggest that these factors do not completely
explain the discrepancy. Secondly, another study found a genetic
predisposition for hysterectomy as indicated by a two fold higher twin
pair correlation for hysterectomy in identical versus fraternal twins.
Thirdly, there exists a rare heritable form of uterine fibroids in
association with fibroids of the skin called Reed's syndrome. Finally, a
recent Russian studies suggest that women with a family history of
fibroids are twice as likely to develop fibroids than women with no
family history. Unfortunately, few scientific studies directly examine
the genetic component of fibroid development.
Recently, researchers at the
Center for Uterine Fibroids have identified mutations in two genes,
HMGI(C) and HMGI(Y), that appear to be important in the development of
some fibroids. (For more information about the genetics of fibroids,
please see articles published
about these genes on the "Publications" page.) Normally,
these genes code for proteins that help control cell growth by
indirectly regulating DNA transcription.
However, mutations in these
genes are probably secondary changes in already genetically susceptible
cells. Therefore, it is likely that other gene(s) crucial for fibroid
development exist that have not yet been identified. To this end, the
staff at the Center for Uterine Fibroids is studying families with at
least one pair of siblings affected by fibroids to search for gene(s)
that predispose women to fibroid development. For information about
this study, including participation, please see, Finding
Genes for Fibroids, on the "Current Studies" page.
Ultimately, understanding
the hormones, growth factors, and gene(s) involved in the formation and
growth of fibroid tumors may lead to innovative, less invasive treatment
options.
Back to top
|
Definition, prevalence and causes
Adenomyosis is a benign
disease of the uterus in which components normally limited to the
endometrium (the thin innermost uterine layer) are found within the
myometrium (the middle muscular layer of the uterus). The exact
prevalence of adenomyosis is not known because the diagnosis can be made
only by microscopic examination of uterine specimens obtained during
surgery or, less often, during biopsy. Some studies estimate that 20% of
women have adenomyosis; however, with careful microscopic analysis of
multiple myometrial samples from an individual uterine specimen, the
prevalence increases to as high as 65%.
The cause of adenomyosis is
also unknown. The most widely accepted theory of adenomyosis development
postulates that the barrier between the endometrium and myometrium,
which normally prevents invasion of endometrial glands and stroma into
the myometrium, is compromised allowing invasion to occur. This process
is thought to occur only in the presence of estrogen, however, little
scientific evidences exists to support this hypothesis.
Adenomyosis most commonly
affects women between the ages of 40 and 50 years and is associated with
a past history of childbirth. Approximately 80% of women with this
disorder have given birth. However, the incidence of adenomyosis does
not correlate with increasing number of pregnancies.
Adenomyosis is also
associated with other uterine disorders. More than 80% of women with
adenomyosis have another pathologic process in the uterus; 50% of
patients have associated fibroids (benign smooth
muscle tumors of the uterus), approximately 11% have endometriosis (endometrial
tissue outside of the uterus, most commonly in the ovaries), and 7% have
endometrial polyps (benign outgrowths of
endometrial tissue). The symptoms of these associated conditions often
obscure the diagnosis of adenomyosis.
Symptoms and diagnosis
A typical uterus with
adenomyosis is boggy and uniformly enlarged. Approximately 80% of uteri
with adenomyosis weigh more than 80 grams (a "normal" uterus
weighs approximately 50 grams), but it is unusual for a uterus in which
adenomyosis is the only pathologic process to exceed 200 grams.
Symptoms of adenomyosis
include abnormal uterine bleeding and pelvic pain. Approximately 60% of
women with adenomyosis experience abnormal uterine bleeding which
usually manifests as either hypermenorrhea (prolonged and/or profuse
uterine bleeding, also called menorrhagia) or metrorragia (irregular,
acyclic bleeding). Dysmenorrhea (pelvic pain during menstruation) is the
second most common symptom in patients with adenomyosis, occurring in
25% of cases.
A review of the literature
demonstrates that only 15% of cases of adenomyosis are correctly
diagnosed before surgery. The reason for this low percentage of
preoperative diagnosis is two-fold; first, many patients with
adenomyosis are asymptomatic in the absence of other uterine pathology,
and second, the presence of adenomyosis is often overshadowed by
associated pathology (e.g., leiomyomas, endometriosis).
D&C (dilation and
curettage) does not aid in diagnosis. (In this procedure, the cervix is
gradually dilated to allow removal of the uterine lining.) Pelvic
ultrasonography may be suggestive but is not definitive. The usefulness
of other imaging studies such as MRI (magnetic resonance imaging) is
currently undetermined.
Treatment
Areas of adenomyosis do not
lend themselves to local surgical excision. The only definitive
treatment for adenomyosis, therefore, is total hysterectomy (surgical
removal of the entire uterus). Synthetic steroid hormones such as
progestins are not helpful and may actually increase the level of pelvic
pain in some patients. GnRH (gonadotropin releasing hormone) agonists
have been used in a few cases, resulting in a transient decrease in
uterine size, in amenorrhea (cessation of menstrual cycling), and even
in the ability to conceive. Unfortunately, regrowth of the adenomyosis
and recurrence of symptoms are usually documented within six months of
cessation of therapy.
Back to top
|
Definition and prevalence
Endometrial polyps are
localized overgrowths of the endometrium (innermost uterine layer) that
project into the uterine cavity. Such polyps may be sessile
(broad-based) or pedunculated (on a narrow stalk) and rarely include
areas of neoplastic (benign or malignant) growth. Specifically,
adenomatous hyperplasia (benign growth of the endometrium) and
endometrial adenocarcinomas (malignant tumors of the glandular component
of the endometrium), have been reported in only 0.6% of cases of
endometrial polyps.
The prevalence of polyps is
estimated to be 10% to 24% of women undergoing hysterectomy (surgical
removal of the uterus) or localized endometrial biopsy. Endometrial
polyps are rare among women younger than 20 years of age. The incidence
of these polyps rises steadily with increasing age, peaks in the fifth
decade of life, and gradually declines after menopause.
Symptoms and diagnosis
The most frequent symptom of
women with endometrial polyps is metrorrhagia (irregular, acyclic
uterine bleeding), which is reported in 50% of symptomatic cases.
Post-menstrual spotting is also common. Less frequent symptoms include
hypermenorrhea (prolonged and/or profuse uterine bleeding, also called
menorrhagia), post-menopausal bleeding, and breakthrough bleeding during
hormonal therapy. Overall, endometrial polyps account for 25% of
abnormal bleeding in both premenopausal and postmenopausal women.
Endometrial polyps are often
diagnosed by microscopic examination of a specimen obtained after
endometrial biopsy or after D&C (dilation and curettage); in this
latter procedure, the cervix is gradually dilated to allow removal of
the uterine lining. As with submucous fibroids, the
diagnosis of polyps can be missed on physical exam if the uterus is not
distended. Therefore, these lesions are being increasingly diagnosed by
techniques such as ultrasound and hysteroscopy. During hysteroscopy, the
uterine cavity is visualized by introducing a small camera on the end of
a tube (hysteroscope) directly into the uterus through the vagina and
cervix. Hysteroscopy with directed biopsy is particularly helpful in the
diagnosis of small polyps within the uterine cavity. Hysterography, a
technique using X-rays to take pictures of the uterine cavity, is rarely
helpful when polyps are small but may yield suggestive findings (e.g., a
smooth space-occupying lesion) when the polyp is large.
Treatment
The majority of cases of
endometrial polyps are cured by thorough curettage. This technique,
which involves removing the endometrial lining of the uterus, is
especially successful in the post-menopausal age group. However, removal
of polyps or other structural abnormalities may be missed by blind
curettage, therefore, hysteroscopic-guided curettage is often useful.
Back to top
|

